鄭炎松研究小組最近在美國化學會的有機化學雜志上發表的論文(J. Org. Chem. 2009, 74, 5660–5663),被香港科技大學的唐本忠教授在美國化學會的新聞周刊《值得關注的化學》上進行了高度評價, 摘文如下:
Noteworthy Chemistry
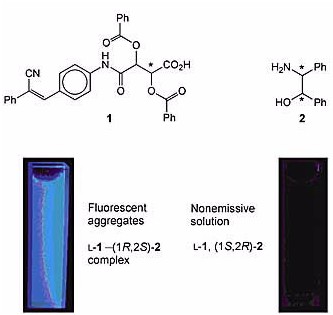
The researchers designed and synthesized a pair of chiral carboxylic acids, D- and L-1, that fluoresce only when they are aggregated. This unique aggregation-induced emission (AIE) attribute allows 1 to act as a chiral fluorescence sensor. For example, L-1 forms enantioselective aggregates with (1R,2S)-2 but not its enantiomeric congener (1S,2R)-2. As shown in the photos, aggregates of the L-1–(1R,2S)-2 complex are visibly fluorescent, but the solution of nonaggregated L-1 and (1S,2R)-2 is nonemissive.
The authors verified the enantioselective nature of the chiral recognition in a control experiment: The D-
1–(1
S,2
R)-
2 complex is fluorescent but D-
1–(1
R,2
S)-
2 is nonemissive. A similar phenomenon occurs when other chiral amines are mixed with the chiral AIE luminogens. The linear relationship between the concentrations of L-
1 and (1
R,2
S)-
2 in dilute aqueous solution permits quantitative analysis of the enantiomer mixtures. (
J. Org. Chem. 2009, 74, 5660–5663;
Ben Zhong Tang)
首段譯文:
用肉眼看手性分子。生命界是手性的王國——幾乎所有基本的生物建筑塊都是手性分子。手性識別技術是非常重要的,但研發這種技術是困難的。一種特別理想的目的是用肉眼觀測手性分子,華中科技大學(中國,武漢)的鄭炎松*和胡于建實現了這種目的。
September 7, 2009
See chiral molecules with the naked eye. The living world is a chiral kingdom—almost all of the basic biological building blocks are chiral molecules. Chiral recognition techniques are very important, but they have been difficult to develop. One particularly desirable goal has been the observation of chiral molecules with the naked eye, and this has now been achieved by Y.-S. Zheng* and Y.-J. Hu at Huazhong University of Science and Technology (Wuhan, China).
